Few contrasts could be more striking than a side-by-side comparison of how the United States and South Korea have fared when confronting the COVID-19 pandemic.
Figures published April 27 by Worldometer and OurWorldData, statistical databases, tell a remarkable tale:
Test, Trace and Isolate
At first glance, South Korea would seem like an unlikely success story. The densely populated country should have been easy pickings for a highly contagious disease. Its 50 million inhabitants occupy an area about the size of Maine. The country also lies in close proximity to China, where COVID-19 originated. Unlike the United States, South Korea lacks prominent medical institutions such as the Centers for Disease Control and Prevention (CDC), the National Institutes for Health (NIH), and the Food and Drug Administration (FDA), along with America’s most renowned hospitals.
On the other hand, South Korea had endured two relatively recent coronavirus outbreaks: SARS in 2003 and MERS in 2015. It got through SARS with just three deaths but struggled to overcome MERS, suffering 38 fatalities. Public health officials sought to learn from their successes and failures in confronting both outbreaks. And when confirmed cases of SARS-CoV-2 began to spike in January and February 2020, South Korean officials adopted a three-step plan to stem the spread: test, trace, and isolate.
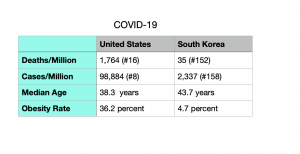
Testing was rigorous and efficient, contact tracing was effective (though it infringed on people’s rights to privacy), and infected patients were quickly isolated from the general population. These measures worked, and the effects were immediate. While much of the world found itself in the grips of the pandemic by spring 2020, South Korea had escaped the worst. As noted by vox.com (April 19), by the end of April, Italy had over 200,000 cases, the United States had surpassed the one-million mark, and South Korea still had fewer than 11,000.
“Adjusted for population, South Korea’s first wave of coronavirus cases was about one-tenth as big as in the United States,” vox.com reports,
What South Korean officials did not do is also instructive. The widespread (if selective) lockdowns that characterized the response in the West were absent from South Korea, where the government never imposed a stay-at-home order. Commuter trains and buses were as packed as in pre-pandemic days, and workers went to offices and manufacturing plants as usual. As a result, South Korea was largely spared the economic and social dislocations—not to mention the loss of life—that have plagued Europe, India, South America, and the United States.
South Koreans do wear masks, just as many did during the SARS and MERS outbreaks. South Koreans also widely adhered to social distancing.
Missed Opportunities in the U.S.
While South Korea’s testing program was up and running quickly, the United States lost precious weeks because of an ill-fated decision by the FDA to place clinical diagnostic testing exclusively in the hands of the CDC.
Instead of gaining knowledge early on about the disease’s severity, lethality, and level of transmission, public health labs “were unable to validate CDC’s contaminated reagents and could not deploy the agency’s tests,” noted Roger D. Klein, M.D., J.D., in a commentary for Health Care News last summer.
Klein, a faculty fellow at the Center for Law, Science, and Innovation at the Sandra Day O’Conner School of Law at Arizona State University, added: “As a result, little testing took place in the United States until the FDA opened the field to private laboratories and in vitro diagnostic test vendors in late February and early March.”
At a hearing last November before the U.S. Senate Homeland Security and Government Affairs Committee, several physicians with first-hand experience in treating COVID-19 patients pointed out that readily-available, at-home treatments could have reduced hospitalizations had their use not been blocked.
“Our government research institutions have invested billions of dollars in expensive patent medication and vaccine development but also nothing in early outpatient treatment,” said Harvey Risch, M.D., Ph. D., a professor of epidemiology at Yale University.
In his testimony, Peter McCullough, M.D., M.P.H., a cardiologist and vice chief of internal medicine at Baylor University Medical Center, cited regulatory barriers and an NIH Guideline that keeps patients from receiving early treatment.
The NIH Guideline allows patients to “sicken at home for two weeks or more, and when finally gasping and choking for air, place them in hospital isolation,” stated McCullough at the hearing.
Bonner R. Cohen, Ph.D., (bcohen@nationalcenter.org) is a senior fellow at the National Center for Public Policy Research.




















