An inexpensive, self-administered antigen strip test could rapidly curb the spread of COVID-19 if government regulators would get out of the way, a Harvard infectious disease expert says.
Michael Mina, M.D., Ph.D., an epidemiologist at Harvard University, and Laurence Kotlikoff, Ph.D., an economist at Boston University, wrote about the idea in a July 3 New York Times op-ed. Mina has been promoting the idea in journals and in a one-hour discussion on August 6 with Med-Cram.
Rapid testing shows much more promise than spending billions of dollars on development of therapeutics or a vaccine, Mina stated in Harvard Magazine on August 3.
“It’s a crapshoot that may or may not work,” Mina writes. “We have solutions, sitting in front of us right now, that are cheaper, would be much quicker to build, and much less risky to actually introduce and roll out.
“The only thing standing in the way is that there just doesn’t seem to be the will to bring a public health tool to market,” Mina writes.
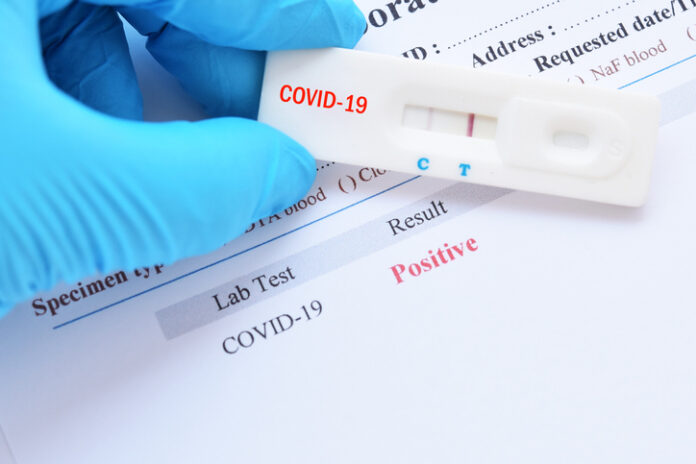
The public health tool is a $1 to $2 self-administered paper strip test, similar to at-home pregnancy tests, that can indicate in a matter of minutes whether an individual is infectious with SARS-CoV2, the virus that causes COVID-19.
The tests are saliva-based and look for antigens to the virus. The tests are half as sensitive as the PCR (polymerase chain reaction) nasal swab tests being used around the country, but what matters is capturing the “threshold of transmissibility,” Mina says.
Concentrating on Transmissibility
A highly sensitive test will pick up particles of the virus when an individual is contagious and a threat to others, but it will also detect evidence of the virus several months after a victim has recovered and is no longer contagious. The strip test, in contrast, picks up the virus only during the period when an individual is likely to transmit it to other people.
Mina gives the hypothetical example of a fire department with an extremely sensitive monitoring system, one that can detect even the smallest fire anywhere in a city. In such a system, the fire trucks would be out on the street headed toward a new destination every time someone struck a match.
“Is that what we want in a fire department?” Mina asked.
Identifying Transmitters on the Spot
To understand how the strip test works, it is helpful to know what a “Ct” value is, says Robert Seheult, M.D.
“It is a way of measuring viral load,” Seheult explained in a MedCram video on July 20. “To be infectious, you have to carry a high viral load.”
Seheult refers in the video to a March 19 article in the New England Journal of Medicine which describes the detected viral load or “Ct value” of infected patients measured against days since the onset of symptoms. Ct values can range from a low of 40 to a high of 15.
“If you’re missing people with higher CT values, who cares?” Mina asks in a clip in Seheult’s presentation. “They are not infecting people.”
“It is not important for a supersensitive test to be given on everyone,” Mina says in the video. “The less-sensitive test [the strip test], one that detects virus at lower Ct values, is fine because it captures the window of transmissibility.”
If someone gets a positive on a strip test, he or she can stay home, which could help control the spread of the virus. Frequency in conducting the tests is much more important than sensitivity, says Mina.
The PCR test, by contrast, must be administered by a health care professional, costs $25 to $200, and can take up to seven days to deliver results, by which time the diagnosis is likely to be of limited value.
Holdup at FDA
Regulatory hurdles at the U.S. Food and Drug Administration (FDA) are the main reason rapid strip tests are not widely available, Mina says.
“What they are expecting is an at-home test that meets all the metrics and milestones of a lab test,” Mina said in the video.
Regulators also want tests to have a system for reporting all results to health departments, but this would require some effort on the part of the people being tested, says Mina.
“So then the companies have to develop some kind of software on the internet for reporting, and we can’t realistically create a test where people are going to report their results each and every time, and this will make the test more expensive,” Mina said.
Mina says the technology is available for a strip test, but few companies want to launch if they will run a high risk of being shut down by regulators or held liable for outcomes of incorrect results.
“We’re seeing some lack of urgency [at the FDA],” Mina said. “Part of it is, who wants to be liable?”
On August 26, FDA granted Emergency Use Authorization to Abbott Laboratories’ for its rapid, antigen test. The “Binax NOW COVID-19 AG Card” rapid test, has an estimated cost of 5 dollars and can give results in 15 minutes. Abbott will ship millions of the tests starting September. On August 27, the Trump Administration announced it purchase 150 million rapids tests to be distributed throughout the country.
The test measures virus proteins by use of a nasal swab, which means it has to be administered by a health care professional. The sample is then inserted into the credit-card sized device. The test is not intended for at-home use and is recommended for individuals who experienced COVID symptoms in the past 7 days.
AnneMarie Schieber (amschieber@heartland.org) is managing editor of Health Care News.
Internet Info:
“How to Fix COVID-19 Testing; Q/A with Dr. Michael Mina: Cheap, At Home, Rapid Antigen Tests,” August, 5, 2020: https://www.youtube.com/watch?v=3seIAs-73G8
“Coronavirus Pandemic Update 98: At Home COVID-19 Testing—a Possible Breakthrough,” July 20, 2020: https://www.youtube.com/watch?v=h7Sv_pS8MgQ&feature=youtu.be
Rapid COVID Test Could be More Complex in Practice: Interview, The Heartland Daily News, August 25, 2020: https://heartlanddailynews.com/2020/08/rapid-covid-test-could-be-more-complex-in-practice-interview/




















[…] The idea of a rapid, frequent and more widespread test for COVID-19 could be more complex in practice, says Roger Klein, M.D., J.D. an expert in molecular diagnostics and regulatory issues and policy advisor to The Heartland Institute, which co-publishes Health Care News. Klein spoke with Health Care News about the hurdles a strip test would have to overcome for it to be a realistic tool in curbing the pandemic. See related article, August 24. […]
[…] antigen test can be performed rapidly, and although it can be far less accurate than the PCR test, Michael Mina, M.D., a professor of epidemiology at Harvard University, says it is sensitive enough to pick up the most infectious cases, a key in controlling a […]
[…] test can be performed rapidly and while it can be far less accurate than the PCR test, experts like Michael Mina, M.D., a professor of epidemiology at Harvard, says it is sensitive enough to pick up the most infectious cases, a key in controlling a […]