Lawmakers are taking action to assure drug freedom for patients and doctors.
State and federal legislators are moving to ensure the rights of physicians and patients to decide what drugs to use.
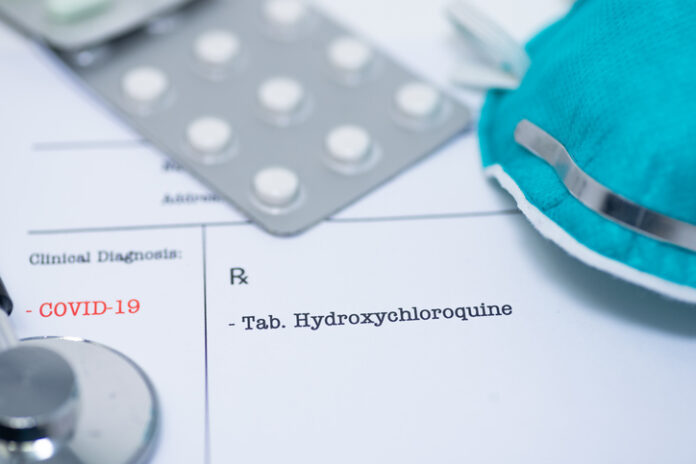
Thirty-one states have either proposed or passed legislation to ensure off-label prescribing rights, with some states specifically mentioning the antiparasitic drug ivermectin or hydroxychloroquine, the antimalarial drug with which President Donald Trump was famously treated off-label.
Tennessee made ivermectin available as an over-the-counter drug upon consultation with a pharmacist, in June. In most states, only physicians can prescribe ivermectin, and they must do so in a heavily politicized environment in which public pressure by governments and medical boards discourages the use of the antiparasitic drug.
In February, Sen. Ron Johnson (R-WI) introduced the Right to Treat Act to prevent federal health agencies from interfering with doctors’ and patients’ treatment decisions.
Feds Suppressed Meds
During the pandemic, regulatory authorities suppressed medications that could work as therapeutics for COVID-19, such as ivermectin. A page on the U.S. Food and Drug Administration’s website falsely claimed ivermectin is “not an anti-viral” and suggested off-label use of the drug is dangerous.
Raising the pressure on doctors, the Federation of State Medical Boards followed the feds’ lead by warning physicians risk losing their licenses for spreading misinformation as the organization defines it.
‘Right to Treat’ Offered
Johnson’s Right to Treat Act would prevent the federal government from influencing such decisions.
“Numerous physicians have had their licenses revoked or threatened to be revoked because they prescribed off-label treatments for COVID-19,” Johnson said in an email to Health Care News.
The proposed law would prevent federal agencies from regulating the practice of medicine and would ensure no federal law, rule, regulation, or policy interferes with the distribution of FDA-approved drugs or Right to Try drugs.
Although guidance from public health agencies regarding off-label use of FDA-approved drugs has been technically nonbinding, the reality has been far different, says Johnson.
“This ‘guidance’ from federal health agencies essentially serves as de facto regulation for states and state boards to implement,” said Johnson. “Federal health agencies have used tweets, media appearances, and other guidance to influence state policies regarding the practice of medicine.”
Boards, Governments Muscling In
Several medical associations, the Federation of State Medical Boards, and individual state medical and pharmacy boards responded to this guidance “by issuing statements in opposition of certain off-label treatments or by sending letters threatening the licenses of physicians and pharmacists that order, prescribe, or dispense certain off-label treatments,” said Johnson.
“Doctors should be at the top of the treatment pyramid,” said Johnson. “However, during the COVID-19 pandemic, aggressive actions by medical and pharmacy boards have restricted a physician’s ability to practice medicine
“Guidance from federal agencies should not prevent doctors from treating their patients, yet that’s exactly what happened,” said Johnson.
The use of an emergency to clamp down on the rights of patients and physicians is the opposite of what should happen, says Johnson.
“Allowing doctors to practice medicine and use their full ‘off label’ prescription rights is particularly important during a pandemic caused by a novel disease for which there are limited or no known treatment options,” said Johnson.
Off-Label Treatment Hindered
The Congressional Research Service finds 12 to 38 percent of all doctor-office prescriptions are off-label. One downside of making off-label prescriptions harder to obtain during the pandemic was the increased difficulty of treating patients with non-COVID problems, says Johnson.
“Hydroxychloroquine is used in an off-label capacity to treat lupus,” said Johnson. “During COVID-19, many of these patients had a hard time filling their prescriptions due to government and medical board barriers to treatment.”
Naomi Lopez, vice president for health care policy at the Goldwater Institute, says laws should always serve the needs of patients, not just during a pandemic.
“Laws that respect the important principle of patient autonomy serve to get us closer to the goal of getting the right treatment to the right patient at the right time,” Lopez said.
“Regardless of the timing—during a public health emergency or not—a patient’s autonomy should never be compromised or limited by arbitrary federal rules and red tape that keep them from seeking needed care,” said Lopez.
Popular Legal Solution
The Right to Treat Act is one of several medicine policy reforms offered since Trump signed the Right to Try law in 2018. Lopez says 41 state and federal Right to Try laws have been enacted to allow patients access to “investigational treatments that have passed basic safety evaluation and remain in clinical trials, without first having to beg the federal government for permission.”
“The original Right to Try law is saving lives, accelerating clinical development, and restoring the practice of medicine where it rightfully belongs: between doctor and patient, not federal bureaucrats,” said Lopez.
Press for Prescribing Rights
The Goldwater Institute is backing an extension of Right to Try, called Right to Try 2.0.
“The Right to Try for Individualized Treatments, also known as Right to Try 2.0, allows patients, under their doctors’ care, to seek personalized treatments that are tailor-made for them, often based on their unique genetic makeup,” said Lopez.
With Right to Try laws defending patient rights, Right to Treat is focused on the other side of the coin: doctors’ prescribing rights. Lopez says the federal government does not have the constitutional authority to intervene in doctors’ decisions.
“The Goldwater Institute affirms that the practice of medicine is an authority granted to state governments,” said Lopez,. “No federal agency or rule should restrict the lawful practice of medicine—including but not limited to the prescribing of FDA-approved treatments and, where legal, investigational treatments.”
Harry Painter (harry@harrypainter.com) writes from Oklahoma.